Pragmatic Trials

A primary goal of our medical school is to support researchers in designing and conducting high-quality randomized trials funded by peer-reviewed agencies. We know that achieving this requires robust clinical trials experience and support.
In 2022, Western was awarded a major external grant from the Canadian Institutes of Health Research to launch a national training program in clinical trials. This program focuses on the core elements of trial design and implementation, with an emphasis on multi-centre pragmatic trials.
With additional funding from the Ontario government, this program has also been able to develop and teach leading practices for patient partnerships in trial teams.
All educational materials are open access and freely available to the research community.
→ Explore the Pragmatic Trials Training Program
To further support our faculty, we offer not-for-profit services for trial leaders and teams looking to design and execute pragmatic trials.
→ Learn more about Pragmatic Trial Services
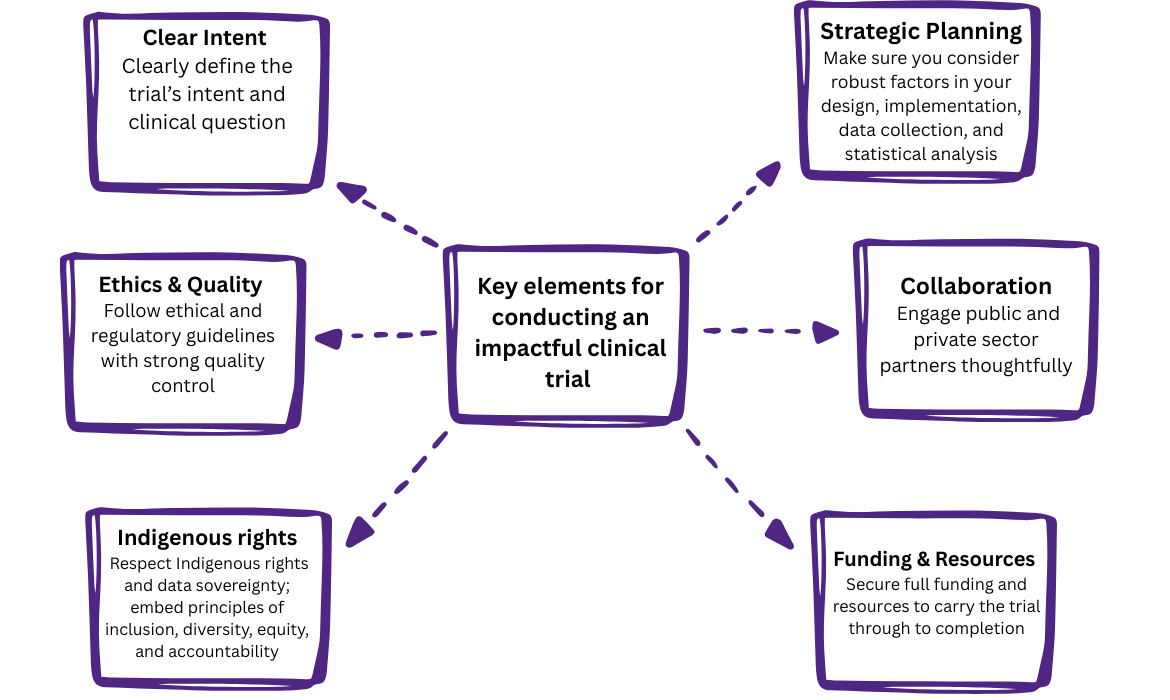
Designing and completing a successful trial requires attention to multiple critical elements: