Failing Faster
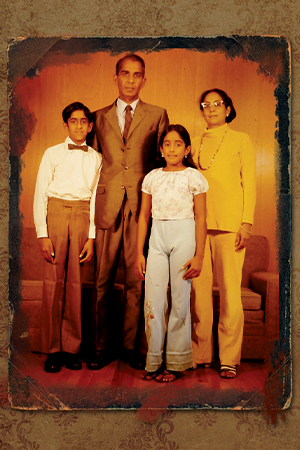
How can we make it less expensive and risky to develop cures for diseases like dementia, Alzheimer’s and Parkinson’s? That’s the question Ravi Menon and his all-star team are pursuing. And their mission is intensely personal.
For a while, it was FaceTime calls.
From his Vancouver nursing home, Kochu Menon would tap his son’s name on his iPhone and wait for his face to appear on the screen.
With 4,000 kilometres between them, the weekly chats were bright spots in the 95-year-old’s routine; a lifeline that connected him to his son and everything he used to be.
Then the calls stopped.
“He doesn’t remember how to use FaceTime any-more,” said Ravi Menon, PhD, scientific director of the Centre for Functional and Metabolic Mapping at Western University.
“I called him last week on his birthday and he had no idea what day it was.”
It’s strange for him, listening as the elder Menon – a former Harvard astrophysicist obsessed with unravelling the mysteries of the universe – struggles for words. And it’s not any easier with his mom, Rama, now 93.
She suffers from tremors and memory loss, likely the results of a Parkinson’s-like condition, and is mostly confined to her bed. A far cry from the driven, PhD-educated electrical engineer she once was – one of North America’s first women ever to achieve the designation.
“It’s sad,” Menon said softly, losing a little of the emotional detachment so often ascribed to scientists, as he clicked through digitized photos of his childhood on a spring day in his office.
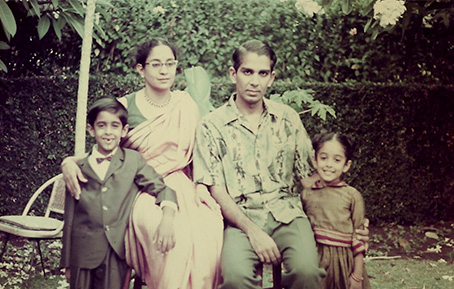
 Top: Ravi Menon with sister, Kusum, and parents Kochu and Rama. Bottom: Menon with his mother Rama.
Top: Ravi Menon with sister, Kusum, and parents Kochu and Rama. Bottom: Menon with his mother Rama.
The images – many taken by his father – illustrate a childhood in several acts, the result of his father’s uncompromising pursuit of his academic interests across countries and continents.
Menon and his sister, Kusum, born just one year apart, with relatives in Bombay, India. The kids and their friends, dressed in Hawaiian leis, celebrating the end of a school year in Honolulu. Hugs in front of a Christmas tree in West Virginia. The children posing in gardens, always tended lovingly by his mom, wherever her husband’s academic journey landed them in the world.
Now Menon’s parents share a nursing home.
It’s a story all too familiar for many Canadians. More than half a million are currently living with dementia alone, a number that’s expected to double by the end of the decade.
And not even someone like Menon – a globally recognized scientist specializing in using MRI to study neurodegenerative diseases – has a solution.
There are no viable treatments to slow down these memory-stealing thieves; no conclusive answers around why the brain is a safe haven for disease to take hold, wreaking havoc on functions like attention, decision-making and planning.
But he’s working on it.
Menon has mobilized a team of world-leading experts in physics, neuroscience, imaging, philosophy and technology that is poised to speed up the race for treatments to slow or stop diseases like Alzheimer’s, Parkinson’s, Lewy body dementia and ALS.
It’s too late now for his mom and dad. But it might not be too late for his generation.
“We’re doing something nobody’s ever done before. We’re creating a one-stop shop to help us identify and predict the success of drugs in human trials faster and cheaper.”
— Ravi Menon
On the team Zoom call on this sunny June afternoon, it’s mostly administrative details at hand.
Menon and his collaborators have won a massive federal grant – the kind specifically geared toward “high risk, high reward” projects that have the potential to transform human health.
The team is made up of powerhouse researchers from Western University, the Centre for Addiction and Mental Health, the University of British Columbia, McGill University and the University of Toronto. They have $24 million for their novel plan to fast-track drug development to treat neurodegenerative diseases – and there’s a lot of paperwork to get through.
This is the boring part for Menon, described as a “relentless, dogged” scientist, by his wife of 30 years, Anne. He’s eager to get to the science.
His specialty is applying physics to neuroscience, working with Canada’s most powerful MRI systems to explore brain function in minute detail. And even for a career scientist who was a key member of a team that demonstrated functional MRI in his late twenties – leading to whispers of a Nobel Prize – this project is shaping up to be his life’s opus.
“We’re doing something nobody’s ever done before,” said Menon of the TRIDENT project, which stands for TRanslational Initiative to DErisk NeuroTherapeutics. “We’re creating a one-stop shop to help us identify and predict the success of drugs in human trials faster and cheaper.”
The problem is simple, but enormous in scale. Drug development is very expensive, particularly when it comes to treatments for diseases that affect cognition, which is notoriously difficult to study. It can cost more than $50 million to bring a single compound to clinical trial and more than $1 billion to gain market approval.
“Even though we’ve ‘cured’ Alzheimer’s in mice a hundred times, inevitably, when you get a treatment to the stage where you’re testing it in clinical trials in humans, it fails,” said Menon, professor of Medical Biophysics and Medical Imaging at Schulich Medicine & Dentistry. “Because of these high rates of failure, and the astronomical costs, many pharmaceutical companies are opting out of drug development in this space. The result is that there are very few therapies in the pipeline to tackle the immense problem of neurodegenerative diseases.”
TRIDENT’s goal is to ‘de-risk’ the drug development cost, by doing better, more efficient testing on new drugs before the clinical trials phase – essentially, enabling them to predict, with far greater accuracy, whether a therapy will be successful in humans, before actually testing it in humans.
To do this, they’re bringing together a series of cutting-edge tests to evaluate how potential therapies impact cognition – including skills like memory, learning, thinking skills and planning – from cell cultures to animal models.
“So much time is wasted by not sharing knowledge, when we could be learning from failures and trying new things. We need to fail faster and move on.”
—Ravi Menon
They’ll probe the brain’s inner workings at a molecular level with unparalleled precision, using some of the world’s most powerful imaging machines. They’ll analyze special lab-grown cells that mimic the human brain – so called “brains in a dish”. They’ll explore how sex plays into neurodegenerative diseases. And they’ll leverage Western University-led technology that’s on the forefront of cognition testing worldwide.
“Determining whether a drug impacts cognition is very challenging, but ultimately, that’s what we want to fix when we’re looking at diseases like Alzheimer’s or Parkinson’s,” said Menon, a Robarts Research Institute scientist. “Our team has the tools to assess cognition in a way that will help us predict with great accuracy whether a drug will succeed in humans.”
This includes using a Western-developed platform – called MouseTRAP – to test cognition in the same way in both animal models and humans, giving a much better indication of how new therapies will likely impact human cognition. Using iPad touch-screens with prompts and rewards, the team can map complex cognitive processes like decision-making and attention and planning across species, to help them assess drugs in ways that have never been attempted before.
Nowhere else in the world offers such an expansive suite of testing, cutting-edge infrastructure and scientific expertise under one collaborative umbrella, said Menon, adding, “This is going to save a lot of time and money.”
Six years. That’s the length of the $24-million Government of Canada New Frontiers in Research Fund grant supporting the team’s groundbreaking research.
It’s also the amount of time within which the team is aiming to identify new treatments they believe will address neurodegenerative diseases in humans. “We think we can do it,” said Menon, glancing over as his constant companion, a six-year-old German Shepherd named Xander, snoozed in a nearby chair in his office.
Not that he’s afraid to fail.

Since he was young, Menon has been asking questions and taking risks, fueled by unyielding curiosity. He’s had a lifelong passion for taking things apart, “tinkering” as he puts it – radios, watches, you name it. Driven to understand what’s inside, as much as his own father ruthlessly tracked a greater understanding of what’s “out there,” in the universe.
These days, he’s probing the inner workings of the world’s most formidable MRI machines, when he’s not tinkering with the old Porsche he’s restoring in his garage.
“The key to doing interesting science is, you don’t know what the answer is,” he said, laughing. “Good experiments fail. If you know what the answer is, that’s not interesting.”
It’s a philosophy that extends to the TRIDENT team’s commitment to a radically open science approach.
“We’re going to share the successes, and the failures, too,” Menon said. “So much time is wasted by not sharing knowledge, when we could be learning from failures and trying new things. We need to fail faster and move on.” The team’s time-and-money-saving approach is already attracting significant interest from the pharmaceutical industry, and they’ll begin running three new compounds through rigorous testing this fall.
Identifying effective treatments for neurodegenerative diseases is an immensely personal mission for many on the team, he said.
“Almost every one of us has someone in our immediate circle who has Alzheimer’s, Parkinson’s, ALS or dementia,” said Menon, a fact that prompted the team to open their pitch for the grant with a slide filled with photos of their affected loved ones.
“The team is incredible,” he said. “If anybody can do it, we can do it.”
BY THE NUMBERS
- $293 BILLION estimated combined direct and indirect costs of dementia in Canada by 2040
- $16.6 BILLION estimated annual Canadian healthcare costs for those affected by dementia by 2031
- $1 BILLION typical cost to move a drug forward to market approval
- $50 MILLION typical cost to bring a single compound to a clinical trial for testing
- $13 MILLION cost of the two powerful MRI machines used by the TRIDENT team
- 4 MILLION expected cases of Alzheimer’s disease and other dementias among Canadians by 2031
- 24 number of faculty collaborators working on the TRIDENT team
- 6 YEARS the team’s timeline for uncovering promising new treatments they believe will work in humans
- 8% of those living with dementia in Canada in 2020 were women
- ZERO cures for neurodegenerative diseases








