Dr. Michael B. Boffa

Associate Professor
Ph.D., Queen's University
Postdoctoral Scholar, Queen's University
Office: Robarts Research Institute - Room 4245A
Phone: 519-931-5222
E-mail: mboffa@uwo.ca
Research Interests
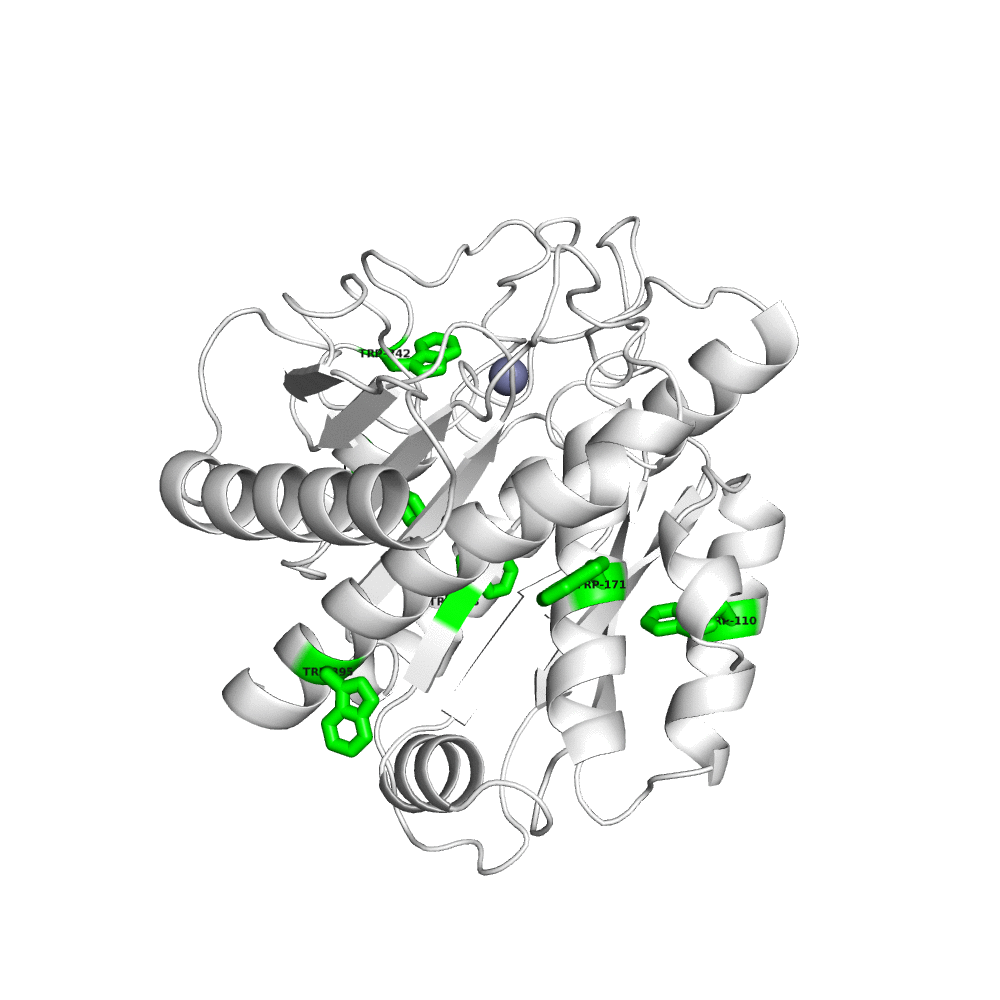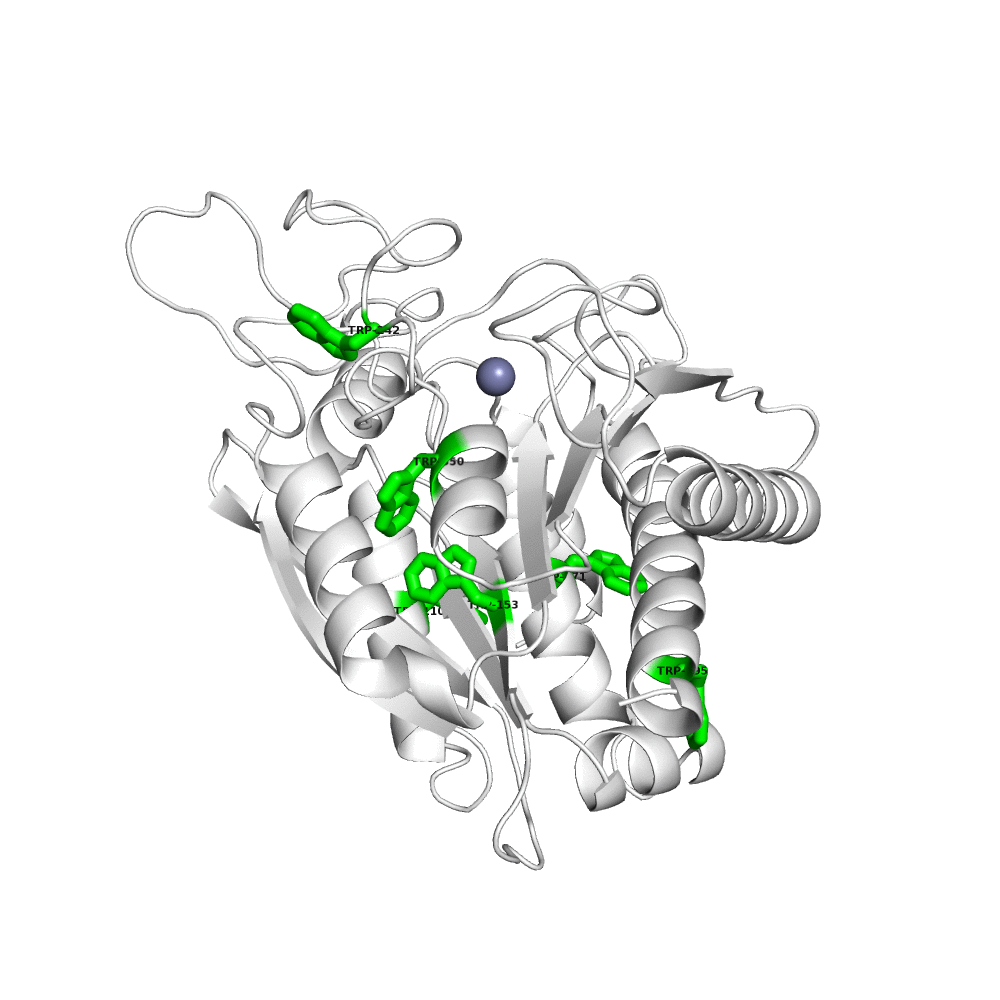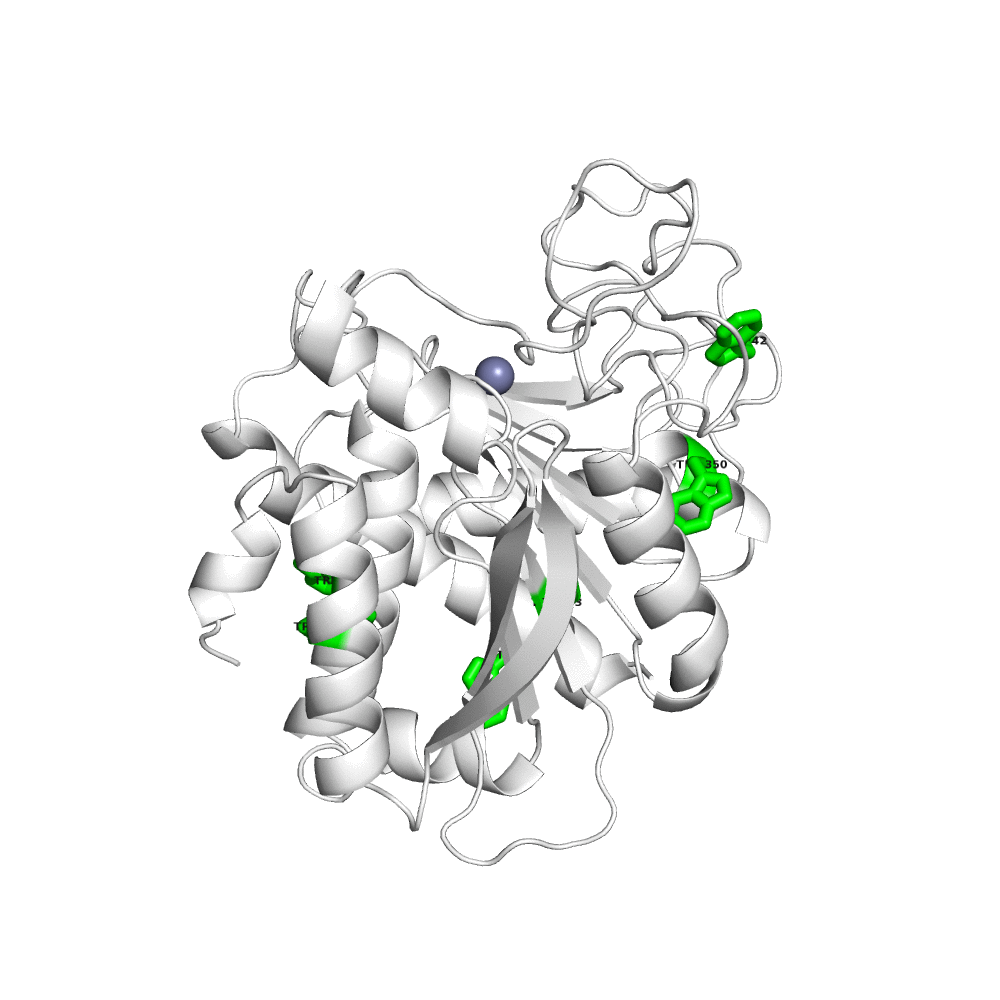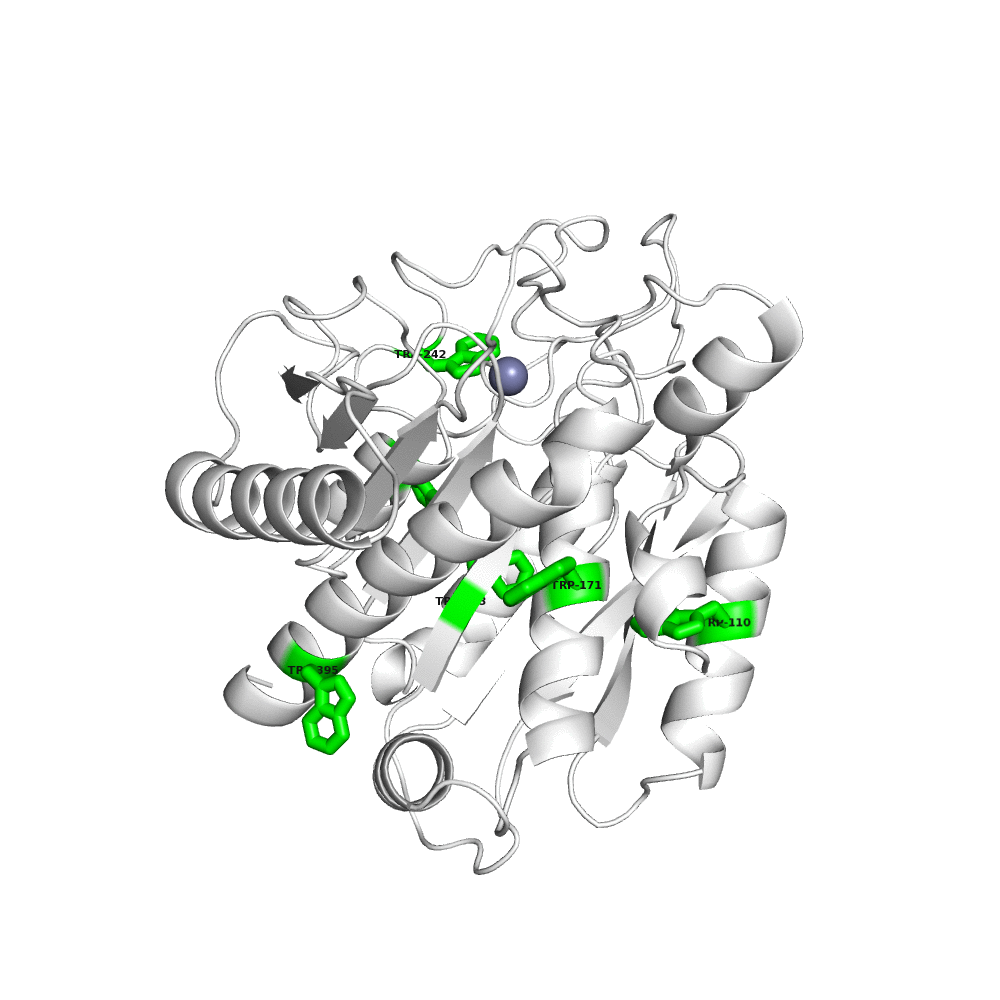
Work in my laboratory largely concerns a fascinating protein called TAFI (thrombin-activatable fibrinolysis inhibitor). TAFI is a protein found in blood plasma with many different roles in both health and disease as well as several very interesting biochemical properties. TAFI is a basic carboxypeptidase zymogen evolutionarily related to the pancreatic carboxypeptidases, and is therefore the precursor of an enzyme that removes carboxyl-terminal lysine and arginine residues from protein and peptide substrates. As its name implies, TAFI is a factor that is activated by the coagulation system, through the actions of an enzyme called thrombin, to form activated TAFI (TAFIa), which then goes on to stabilize blood clots. Additional functions of TAFI have been documented in inflammation, tissue fibrosis, and wound healing. My own laboratory has recently discovered that TAFI also inhibits the metastatic behaviours of cancer cells.
My research group is pursuing studies of structure/function relationships in TAFI, using molecular biological and protein biochemical approaches. We are particularly interested in a hallmark feature of TAFIa: unlike most proteins, the enzyme is intrinsically unstable and the spontaneous loss of its activity is a key regulatory property. We also have a major focus on the role of TAFI in breast cancer metastasis, which we are pursuing using biochemical, cell biological, and animal model approaches. Finally, we have made key contributions to the understanding of the mechanisms of regulation of the gene encoding TAFI, and we are continuing to pursue new avenues of research in this realm.
Publications
See list of publications from PubMed.








