Shipping Instructions
Blood samples for in vitro testing:
IMPORTANT NOTE: Please call Nancy Friedrich at 519 931 5777 Ext 24209 before you ship the samples
- Thirty millilitres (20-30 mL) of peripheral blood must be withdrawn from patient by venipuncture and collected in heparinized sample tubes (Sodium heparin - dark green cap collection tubes) on the day of shipping.
- An equal amount of sample from local healthy volunteer (control) should also be provided. We will provide another internal control to monitor for any handling discrepancies.
- Ship samples at room temperature. DO NOT FREEZE SAMPLES .
- The shipment should be arranged to arrive at the DSLab on Monday or Tuesday of each week.
- If shipment takes place during the winter months or by air, please make sure the tubes are packaged in such a way to prevent freezing (e.g., wrapped in absorbent wadding)
- The test results along with a report will be sent to the referring doctor within two weeks of receiving the samples.
Please email or call (leave a voice message) informing us that blood samples have been shipped and the estimated arrival date/time. If you have any questions, please call the DSLab at 519 931 5777 Ext. 24209 or email aelzaga@uwo.ca.
Hair samples for cortisol analysis

Please contact us before sending any samples. Samples will only be analyzed with a completed Sample Collection Form (See below).
STEP 1
GATHER METERIALS FOR SAMPLE COLLECTION:
You will need:
- Willing subject
- Sharp, clean scissors
- Printed DSLab Hair Collection Form (See link below to download)
- Clear scotch tape
- Gloves (optional)
- Hair clip (optional)
- Pen
STEP 2
LOCATE THE POSTERIOR VERTEX REGION OF THE SCALP:
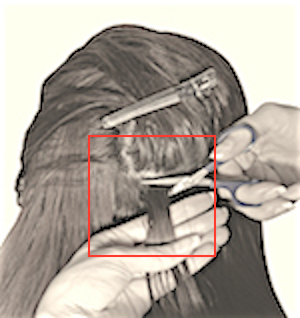
Why the posterior vertex?
This region of the head has the most consistent hair growth rate. Sampling from here will minimize the variation in sample measurements.
STEP 3
ISOLATE HAIR WITH HAIR CLIP (OR FINGERS) & CUT HORIZONTALLY WITH SCISSORS AS CLOSE TO SCLAP AS POSSIBLE.
How much hair is required?
~100 strands of hair (5mm in diameter; thickness of pencil eraser).
PLEASE MAKE SURE THAT HAIR STRANDS ARE ALIGNED (AND SECURED WITH TAPE) TO THE DIRECTION OF THE SCALP AND THAT THE DIRECTION OF THE SCALP IS CLEARLY INDICATED (USE THE COLLECTION FORM PROVIDED BELOW)
How long should the hair be?
This is dependent on the required timeframe. Human human hair grows at a rate of ~ 1cm/month so the sample length should reflect this rate (i.e. 8 months≈ 8 cm).
STEP 4
TAPE THE SCALP END OF THE HAIR TO THE HAIR SAMPLE COLLECTION FORM & FILL OUT STUDY ID, SUBJECT ID, & COLLECTION DATE.
What kind of tape should be used?
Clear scotch tape works best. It is easily removed and leaves no residue on the hair.
How should the sample be stored?
Fold the paper along the length of the hair & place in envelope to secure the sample. Store sample in a dry location at room temperature until shipment.
Please follow the instructions in the Hair Sample Collection Form. To download the pdf click HERE.
Click HERE to download the Hair sample intake form








