Young researchers on frontlines of brain studies among recipients of early career funding

By Cam Buchan
Taylor Schmitz is using the same strategy to conquer Alzheimer’s that the disease uses in attacking the human brain – he’s coming after the neurological disorder with a multiple-pronged approach.
“Unfortunately, advanced Alzheimer’s is very difficult to treat due its complexity,” said Schmitz. “By the time a patient begins to show the earliest signs of memory loss, multiple different types of pathology in the brain will have already snowballed together.”
Many current treatment strategies only tackle one symptom at a time, as opposed to the entire “snowball,” or simply attempt to support what remains of the intact brain once it succumbs to the disease. Approximately 1 million Canadians currently live with Alzheimer’s disease-related dementia.
“To find a cure, we need to better understand this disease at earlier and less complicated stages of its progression, long before memory loss starts,” said Schmitz, PhD, from the Department of Physiology and Pharmacology.
Schmitz, along with Jibran Khokhar, PhD, from the Department of Anatomy and Cell Biology, are being recognized for their work with Early Researcher Awards (ERA) funding from the Ontario government. The program helps exceptional early-career researchers access the latest technologies, equipment and talent to build teams to advance their research projects. Each award from government is valued at $100,000, and is matched by an additional $50,000 from the researcher's institution.
They are among a record 10 Western researchers to receive the awards.
“Early career researchers bring new ideas, new tools and fresh perspectives that elevate our existing teams to tackle the most enduring health challenges in innovative ways,” said Robert Bartha, Vice Dean of Research and Innovation at Schulich Medicine & Dentistry. “Not only is recruiting and supporting early career faculty a key component of our School’s research strategy, it will accelerate our efforts better therapies and treatment options for patients, which is our ultimate goal.”
Fighting the battle against mental illness and addiction
Khokhar is investigating the terrible toll that mental illness and addiction are inflicting on Ontarians – nearly two times the disease burden of cancer and seven times that of pre-pandemic infectious diseases. At the same time, 50 to 80 per cent of people with mental illness have a co-occurring substance use problem, which often occurs in patients with schizophrenia, making this affliction significantly worse.
“Unfortunately, few treatment options are available to reduce alcohol drinking in these patients,” said Khokhar.
Utilizing animal models, Khokhar will study the neurobiological mechanisms underlying alcohol abuse in patients with schizophrenia. Using brain imaging methods akin to those used in patients, his team will assess whether dysfunctions in the brain’s reward circuit can predict future alcohol intake. They will also investigate ways to alter the brain’s circuitry to see if alcohol intake can be reduced and look more deeply at the impact of an antipsychotic drug known to reduce alcohol drinking on brain reward circuit dysfunction.
“Such treatments will improve patient care, and reduce the high health-care burden associated with substance use in these patients,” Khokhar said.
Enlisting the brain’s own defences
Schmitz, meanwhile, is also looking at Alzheimer’s from a different angle.
He is investigating how the brain’s own defences help in protecting itself against Alzheimer’s disease. For this work, Schmitz and his team have received more than $725,000 in funding from the Canadian Institutes for Health Research (CIHR) Brain Health and Cognitive Impairment in Aging (BHCIA), an initiative that supports research to promote brain health during aging while addressing the needs of people living with dementia and their caregivers.
Using Robarts Research Institute’s state-of-the-art brain imaging and cognitive testing facilities, Schmitz, PhD, and co-investigators Vania Prado and Marco Prado, are studying a drug – named LM11A-31 – that boosts the brain’s repair systems. The drug was developed by Professors Frank Longo and Stephen Massa, researchers based at Stanford University and University of California, San Francisco.
“Recent evidence indicates the brain uses it own systems to fight Alzheimer’s pathology years before symptoms appear,” said Schmitz. “Understanding this process could lead to therapies enhancing the brain's own defenses against the disease.”
Schmitz and his team are looking at the drug’s effectiveness in different stages of the disease and how it interacts with apolipoprotein E (APOE), the most common gene linked to Alzheimer’s disease. While only 15 to 20 per cent of people have the high-risk APOE e4 genotype, it is present in more than half of Alzheimer’s patients.
The research project, Leveraging deep biology for brain resilience to AD pathology, seeks to identify which patients would benefit from LM11A-31 and when is the most beneficial time to administer it.
Working with mouse models developed by co-investigators Vania and Marco Prado – both professors in the Department of Physiology and Pharmacology – the team will compare how the drug works in the APOE e4 and the lower-risk APOE e3 genes.
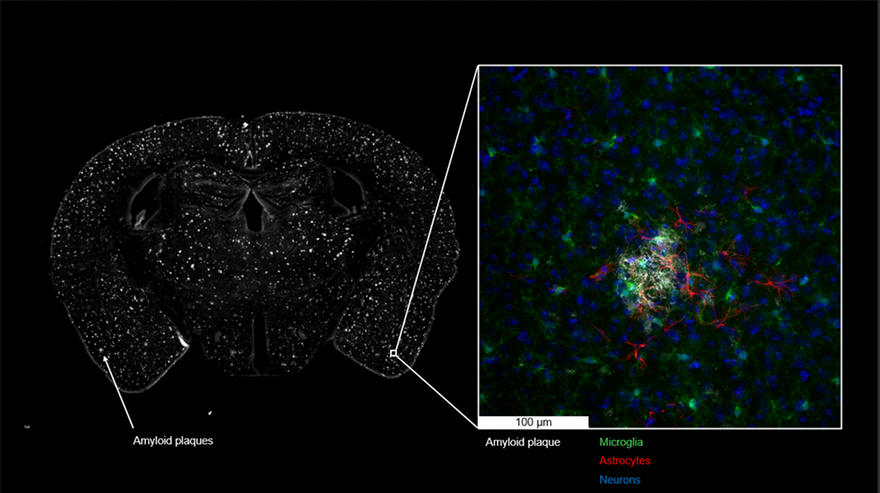
Using brain imaging and cognitive testing strategies that are virtually identical to those used in human trials, the research team will also examine the drug’s effectiveness when administered at different time periods as well as monitor disease progression within the brain.
“This is a key question,” said Schmitz. “We suspect the drug will be most effective when initiated at very early stages of the disease, prior to neuronal loss.”
Schmitz said the team hopes to get a better understanding of how LM11A-31 works to assist the brain’s own repair systems. “These insights will help to optimize the design of future clinical trials evaluating the effectiveness of this drug in humans.
Ultimately, Schmitz hopes the findings from this project will shift research attention to the brain’s natural systems of resilience to aging and Alzheimer’s disease.
“There is a tendency for current research on Alzheimer’s disease to focus on the pathology, loss of neurons and cognitive decline—the bad stuff—to the exclusion of the brain’s remarkable ‘built-in’ capacity for slowing their progression.”
LM11A-31 is under clinical development by PharmatrophiX – a developer of disease-modifying small molecule drugs designed to target mechanisms of underlying neurogenerative disorders.








