Research
Over 90 percent of pregnant individuals take at least one medication during pregnancy, but the exclusion of pregnant individuals from clinical drug trials often means that the risk to both mother and baby is unknown. As a result, pregnant individuals often take medications with limited data to inform safety, dosing, and efficacy.
To address these issues, the Hutson/Garcia-Bournissen lab use an ex vivo placental dual perfusion model and physiology-based pharmacokinetics to study the placental transfer of drugs. The data generated from their research enables physicians to better assess the risk-benefit ratio and provide counseling to patients regarding drug use during pregnancy.
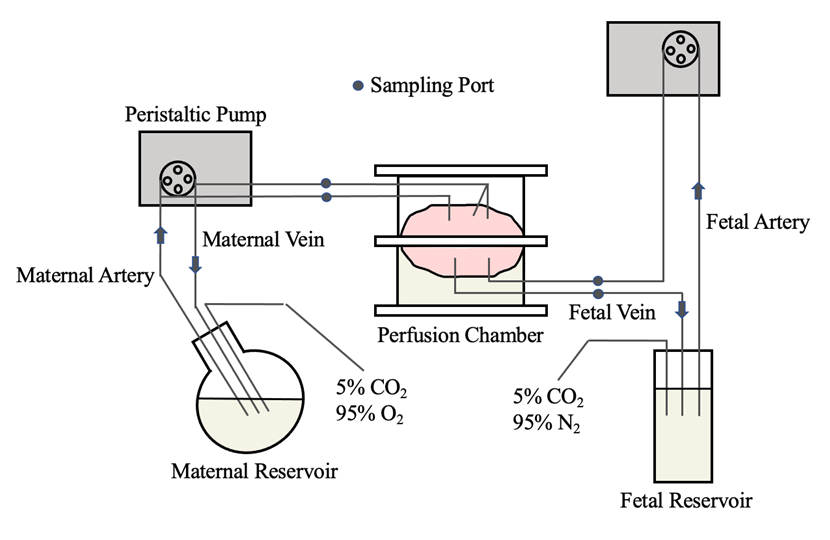
A schematic of our ex vivo placental dual perfusion model







