Research
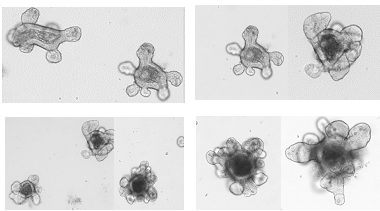
The work currently being carried out in the Asfaha lab includes both in vitro and in vivo models. We are using “mini-guts” derived from animal and human specimens and “grown in a dish”, as well as, transgenic mouse models that have been uniquely created to tag and track specific stem cell populations. Mini-guts are derived from stem cells and form gastrointestinal tissues that are only one cell thick but structurally similar to a normal GI tract.
GI Stem Cells
 The Asfaha lab’s primary interest is in gastrointestinal stem cells with the focus on their role in tissue regeneration and cancer. The lab has been strongly focused on distinguishing amongst the role of various epithelial stem/progenitor cell populations in gut healing. In this regard, we are currently focused on the following two major areas of study:
The Asfaha lab’s primary interest is in gastrointestinal stem cells with the focus on their role in tissue regeneration and cancer. The lab has been strongly focused on distinguishing amongst the role of various epithelial stem/progenitor cell populations in gut healing. In this regard, we are currently focused on the following two major areas of study:
1) In work done while in the Wang laboratory at Columbia University, Dr. Asfaha previously demonstrated that cytokeratin 19 (K19 or Krt19) marks a radiation-sensitive intestinal stem cell population distinct from the classical Lgr5+ stem cells. In work now being carried out in the lab, we are studying what makes K19+ cells relatively radioresistant when compared to Lgr5+ stem cells.
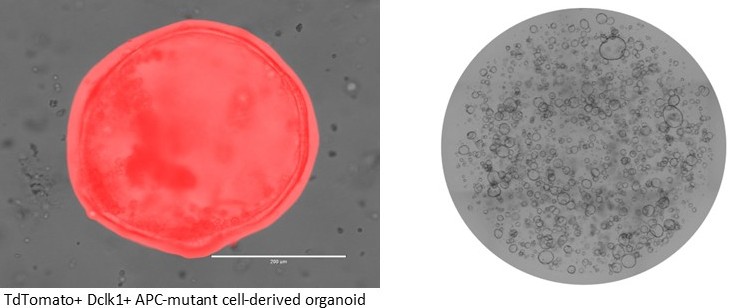
2) Dr. Asfaha has also previously demonstrated that a subset of intestinal cells expressing the protein Dclk1 are long-lived and do not normally proliferate (are quiescent); however, during colitis transform into stem cells that give rise to colon cancer. The Asfaha lab is now also focused on what inflammatory cues allow for the transition of Dclk1+ tuft cells to become stem like.
Asfaha S, Hayakawa Y, Muley A, Stokes S, Graham TA, Ericksen R, Westphalen CB, von Burstein J, Mastracci TL, Worthley DL, Quante M, Rustgi AK, Wang TC. Lgr5(-)/Krt19(+) cells are radioresistant cancer initiating stem cells in the intestine and colon. Cell Stem Cell June 2015; 16:627-38
CB Westphalen, S Asfaha, Y Hayakawa, MD Gershon, M Quante, Wang TC. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest. 2014; 124:1283-1295. PMC3934168
Inflammatory Bowel Disease (IBD)
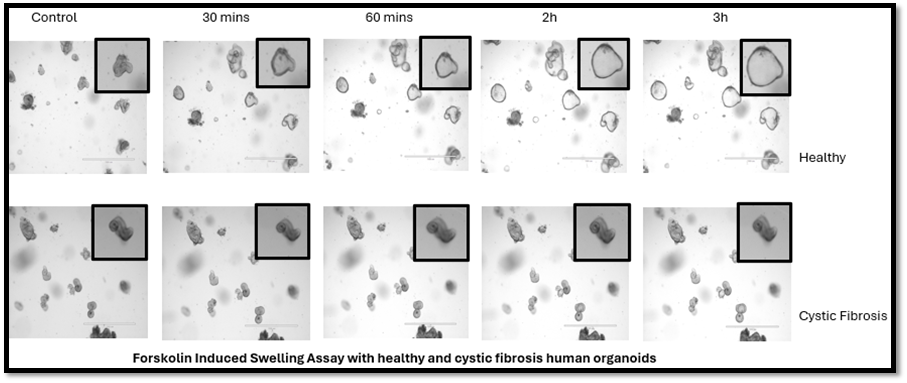
IBD including Crohn’s Disease and Ulcerative colitis, is characterized by chronic inflammation of the gut (colitis) resulting in intestinal injury that presents as abdominal pain, bloody diarrhea, obstruction and fistulizing disease. Despite current therapies, the cause of this disease remains unknown. For this reason, our lab is investigating what regulates intestinal stem cells with the goal of promoting colonic healing following colitis. Specifically, we are studying the role of the intestinal stem cell niche in work being carried out in collaboration with Daniel Worthley’s lab (Adelaide, Australia) who identified a novel mesenchymal stem cell marker (Gremlin1), a BMP inhibitor. In another project recently published (EMBO Journal, 2019), we have collaborated with Ophir Klein’s group at UCSCF, to demonstrate the role of Atoh1+ secretory progenitors in colonic regeneration.
Worthley DL, Churchill M, Compton JT, S Asfaha … Lee FY, Karsenty G, Mukherjee S, Wang TC. Gremlin1 identifies a skeletal stem cell with bone cartilage and reticular stromal potential. Cell. 2015;160(1-2):269-84.PMID: 25594183
D Castillo-Azofeifa, E Fazio, R Nattiv, H Good, T Wald, F.J. de Sauvage, OD. Klein and S Asfaha. (2018). Atoh1+ secretory progenitors possess renewal capacity independent of Lgr5+ cells during colonic regeneration. EMBO Journal 2019; Feb 15;38(4)
Cancer Microenvironment
The Asfaha laboratory is also conducting studies in this area in order to better characterize the role of the intestinal and colonic cancer niche. The lab is studying the role of bone-marrow derived and resident inflammatory cells, as well as cancer-associated myofibroblasts in gastrointestinal tumors. Dr. Asfaha has contributed to investigations of cancer-associated inflammatory cells and fibroblasts in GI cancers ranging from colon cancer to gastric and pancreatic cancer.
XD Yang, W Ai, Asfaha S, G Bhagat, RA Friedman, G Jin, H Park, B Shykind, TG Diacovo, A Falus & Wang TC. Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b+Ly6G+
Dubeykovskaya Z, Si Y, Chen X, Worthley DL, Renz BW, Urbanska AM, Hayakawa Y, Xu T, Westphalen CB, Dubeykovskiy A, Chen D, Friedman RA, Asfaha S, Nagar, K, Muthupalani, S, Fox JG, Kitajewski J, Wang TC. Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nature Communications. 2016 Feb 4;7:10517. doi: 10.1038








