

The Biomolecular NMR Facility is an open access research facility located in the Department of Biochemistry in the Schulich School of Medicine.
The facility houses two high field NMR instruments (Varian INOVA 600 MHz), and a variety of accessories including detection of a wide variety of nuclei, broad sample temperature capabilities and cryogenically cooled high sensitivity hydrogen and carbon probes for enhanced sensitivity.
The facility is available to researchers who wish to conduct and analyze their own NMR experiments, or submit samples on a fee-for-service basis. We are also available to discuss your research project with you and help develop experiments that may address your biological questions.
Our facility serves the Department of Biochemistry and many other departments in the Schulich School of Medicine and Dentistry and Western University, as well as external research institutions and commercial clients.
Some of the capabilities of the facility include:
Characterization of small biological molecules
Identification of new compounds
Enzyme kinetics
Characterization of contaminants
Metabolomics in biofluids
Three dimensional structure determination of soluble proteins in solution
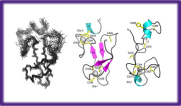 Identification of new protein folds
Identification of new protein folds
Drug and receptor interaction
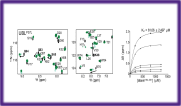 Binding locations on a per residue level
Binding locations on a per residue level
Protein Dynamics
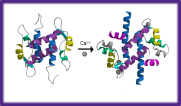 Conformational changes on picosecond-
Conformational changes on picosecond-
Protein Interactions
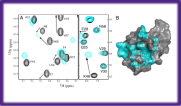 Mapping of protein-protein and protein-ligand
Mapping of protein-protein and protein-ligand
Schulich School of Medicine & Dentistry
Western University
MSB, Rm. 309
London, ON, Canada, N6A 5C1
bionmr@uwo.ca
t. 519.661.3058